Protist
| Protists Temporal range:
| |
|---|---|

| |
| Examples of protists. Clockwise from top left: red algae, kelp, ciliate, golden alga, dinoflagellate, metamonad, amoeba, slime mold. | |
| Scientific classification (paraphyletic)
| |
| Domain: | Eukaryota |
| Supergroups[1] | |
| Cladistically included but traditionally excluded taxa | |
| |
A protist (/ˈproʊtɪst/ PROH-tist) or protoctist is any eukaryotic organism that is not an animal, land plant, or fungus. Protists do not form a natural group, or clade, but are a polyphyletic grouping of several independent clades that evolved from the last eukaryotic common ancestor.
Protists were historically regarded as a separate taxonomic kingdom known as Protista or Protoctista. With the advent of phylogenetic analysis and electron microscopy studies, the use of Protista as a formal taxon was gradually abandoned. In modern classifications, protists are spread across several eukaryotic clades called supergroups, such as Archaeplastida (photoautotrophs that includes land plants), SAR, Obazoa (which includes fungi and animals), Amoebozoa and Excavata.
Protists represent an extremely large genetic and ecological diversity in all environments, including extreme habitats. Their diversity, larger than for all other eukaryotes, has only been discovered in recent decades through the study of environmental DNA and is still in the process of being fully described. They are present in all ecosystems as important components of the biogeochemical cycles and trophic webs. They exist abundantly and ubiquitously in a variety of forms that evolved multiple times independently, such as free-living algae, amoebae and slime moulds, or as important parasites. Together, they compose an amount of biomass that doubles that of animals. They exhibit varied types of nutrition (such as phototrophy, phagotrophy or osmotrophy), sometimes combining them (in mixotrophy). They present unique adaptations not present in multicellular animals, fungi or land plants. The study of protists is termed protistology.
Definition
[edit]Protists are a diverse group of eukaryotes (organisms whose cells possess a nucleus) that are primarily single-celled and microscopic but exhibit a wide variety of shapes and life strategies. They have different life cycles, trophic levels, modes of locomotion, and cellular structures.[3][4] Although most protists are unicellular, there is a considerable range of multicellularity amongst them; some form colonies or multicellular structures visible to the naked eye. The term 'protist' is defined as a paraphyletic group of all eukaryotes that are not animals, plants or fungi.[5] Because of this definition by exclusion, protists encompass almost all of the broad spectrum of biological characteristics expected in eukaryotes.[6]
The distinction between protists and the other three eukaryotic kingdoms has been difficult to settle. Historically, the heterotrophic protists, known as protozoa, were considered part of the animal kingdom, while the phototrophic ones, called algae, were studied as part of the plant kingdom. Even after the creation of a separate protist kingdom, some minuscule animals (the myxozoans)[7] and 'lower' fungi (namely the aphelids, rozellids and microsporidians, collectively known as Opisthosporidia) were studied as protists,[8][9] and some algae (particularly red and green algae) remained classified as plants.[10] According to the current consensus, the term 'protist' specifically excludes animals, embryophytes (land plants) —meaning that all algae fall under this category— and all fungi, although lower fungi are often studied by protistologists and mycologists alike.[1]
The names of some protists (called ambiregnal protists), because of their mixture of traits similar to both animals and plants or fungi (e.g. slime molds and flagellated algae like euglenids), have been published under either or both of the botanical (ICN) and the zoological (ICZN) codes of nomenclature.[11][12]
Characteristics
[edit]Structure
[edit]Protists display a wide range of distinct morphologies that have been used to classify them for practical purposes, although most of these categories do not represent evolutionary cohesive lineages or clades and have instead evolved independently several times. The most recognizable types are:[13]
- Amoebae. Characterized by their irregular, flexible shapes, these protists move by extending portions of their cytoplasm, known as pseudopodia, to crawl along surfaces.[14] Many groups of amoebae are naked, but testate amoebae and foraminifera grow a shell around their cell made from digested material or surrounding debris. Some, known as radiolarians and heliozoans, have special spherical shapes with microtubule-supported pseudopodia radiating from the cell.[13] Some amoebae are capable of producing stalked multicellular stages that bear spores, often by aggregating together; these are known as slime molds.[15] The main clades containing amoebae are Amoebozoa (including various slime molds and testate amoebae) and Rhizaria (including famous groups such as foraminifera and radiolarians, as well as a few testate amoebae).[16][17] Even some individual amoebae can grow to giant sizes visible to the naked eye.[18][19]
- Flagellates. These protists are equipped with one or more whip-like appendages called cilia, undulipodia or eukaryotic flagella,[a] which enable them to swim or glide freely through the environment. Flagellates are found in all lineages, reflecting that the common ancestor of all living eukaryotes was a flagellate. They usually exhibit two cilia (e.g., in Provora, Telonemia, Stramenopiles, Alveolata, Obazoa and most excavates), but there are a number of flagellate groups with a high number of cilia (such as Hemimastigophora and other excavates).[13] Some groups, such as the well-known ciliates and the parasitic opalinids, have a cell surface covered in rows of cilia that beat rhythmically. A few groups of amoebae have retained their flagella, making them amoeboflagellates.[23]
- Algae. They are the photosynthetic protists, and can be found in most of the main clades, completely intermingled with heterotrophic protists which are traditionally called protozoa.[24] Algae exhibit the most diverse range of morphologies, from single flagellated or coccoid cells (e.g., cryptophytes, haptophytes, dinoflagellates, chromerids, many green algae, ochrophytes, euglenophytes) to amoeboid cells (chlorarachniophytes) to colonial and multicellular macroscopic forms (e.g., red algae, some green algae, and some ochrophytes such as kelp).[25]
- Fungus-like protists. Several clades of protists have evolved an appearance similar to fungi through hyphae-like structures and a saprophytic nutrition. They have evolved multiple times, often very distantly from true fungi (e.g., the oomycetes, labyrinthulomycetes and hyphochytrids, in Stramenopiles; the myxomycetes, in Amoebozoa; the phytomyxeans, in Rhizaria; the perkinsozoans, in Alveolata).[26][27]
- Sporozoa. This category traditionally included parasitic protists that reproduced via spores (the apicomplexans, microsporidians, myxozoans and ascetosporeans).[7] Its current use is restricted to the apicomplexans,[28] such as Plasmodium falciparum, the cause of malaria.[29]
Physiology
[edit]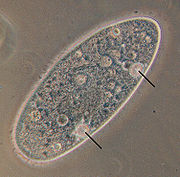
In general, protists are typical eukaryotic cells that follow the same principles of physiology and biochemistry described for those cells within the "higher" eukaryotes (animals, fungi or plants): they are aerobic organisms that consume oxygen to produce energy through mitochondria, and those with chloroplasts perform carbon fixation through photosynthesis in chloroplasts. [30] However, many have evolved a variety of unique physiological adaptations that do not appear in the remaining eukaryotes.[31]
- Osmoregulation. Freshwater protists without cell walls are able to regulate their osmosis through contractile vacuoles, specialized organelles that periodically excrete fluid high in potassium and sodium through a cycle of diastole and systole. The cycle stops when the cells are placed in a medium with different salinity, until the cell adapts.[31]

- Energetic adaptations. The last eukaryotic common ancestor was aerobic, bearing mitochondria for oxidative metabolism. Many lineages of free-living and parasitic protists have independently evolved and adapted to inhabit anaerobic or microaerophilic habitats, by modifying the early mitochondria into hydrogenosomes, organelles that generate ATP anaerobically through fermentation of pyruvate. In a parallel manner, in the microaerophilic trypanosomatid protists, the fermentative glycosome evolved from the peroxisome.[31]
- Sensory adaptations. Many flagellates and probably all motile algae exhibit a positive phototaxis (i.e. they swim or glide toward a source of light). For this purpose, they exhibit three kinds of photoreceptors or "eyespots": (1) receptors with light antennae, found in many green algae, dinoflagellates and cryptophytes; (2) receptors with opaque screens; and (3) complex ocelloids with intracellular lenses, found in one group of predatory dinoflagellates, the Warnowiaceae. Additionally, some ciliates orient themselves in relation to the Earth's gravitational field while moving (geotaxis), and others swim in relation to the concentration of dissolved oxygen in the water.[31]
- Endosymbiosis. Protists have an accentuated tendency to include endosymbionts in their cells, and these have produced new physiological opportunities. Some associations are more permanent, such as Paramecium bursaria and its endosymbiont Chlorella; others more transient. Many protists contain captured chloroplasts, chloroplast-mitochondrial complexes, and even eyespots from algae. The xenosomes are bacterial endosymbionts found in ciliates, sometimes with a methanogenic role inside anaerobic ciliates.[31]
Sexual reproduction
[edit]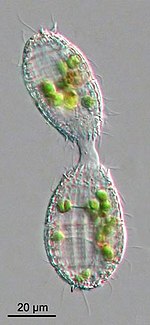
Protists generally reproduce asexually under favorable environmental conditions, but tend to reproduce sexually under stressful conditions, such as starvation or heat shock. Oxidative stress, which leads to DNA damage, also appears to be an important factor in the induction of sex in protists.[33]
Eukaryotes emerged in evolution more than 1.5 billion years ago.[34] The earliest eukaryotes were protists. Although sexual reproduction is widespread among multicellular eukaryotes, it seemed unlikely until recently, that sex could be a primordial and fundamental characteristic of eukaryotes. The main reason for this view was that sex appeared to be lacking in certain pathogenic protists whose ancestors branched off early from the eukaryotic family tree. However, several of these "early-branching" protists that were thought to predate the emergence of meiosis and sex (such as Giardia lamblia and Trichomonas vaginalis) are now known to descend from ancestors capable of meiosis and meiotic recombination, because they have a set core of meiotic genes that are present in sexual eukaryotes.[35][36] Most of these meiotic genes were likely present in the common ancestor of all eukaryotes,[37] which was likely capable of facultative (non-obligate) sexual reproduction.[38]
This view was further supported by a 2011 study on amoebae. Amoebae have been regarded as asexual organisms, but the study describes evidence that most amoeboid lineages are ancestrally sexual, and that the majority of asexual groups likely arose recently and independently.[39] Even in the early 20th century, some researchers interpreted phenomena related to chromidia (chromatin granules free in the cytoplasm) in amoebae as sexual reproduction.[40]
Sex in pathogenic protists
[edit]Some commonly found protist pathogens such as Toxoplasma gondii are capable of infecting and undergoing asexual reproduction in a wide variety of animals – which act as secondary or intermediate host – but can undergo sexual reproduction only in the primary or definitive host (for example: felids such as domestic cats in this case).[41][42][43]
Some species, for example Plasmodium falciparum, have extremely complex life cycles that involve multiple forms of the organism, some of which reproduce sexually and others asexually.[44] However, it is unclear how frequently sexual reproduction causes genetic exchange between different strains of Plasmodium in nature and most populations of parasitic protists may be clonal lines that rarely exchange genes with other members of their species.[45]
The pathogenic parasitic protists of the genus Leishmania have been shown to be capable of a sexual cycle in the invertebrate vector, likened to the meiosis undertaken in the trypanosomes.[46]
Diversity
[edit]
The species diversity of protists is severely underestimated by traditional methods that differentiate species based on morphological characteristics. The number of described protist species is very low (ranging from 26,000[48] to 74,400[47] as of 2012) in comparison to the diversity of plants, animals and fungi, which are historically and biologically well-known and studied. The predicted number of species also varies greatly, ranging from 1.4×105 to 1.6×106, and in several groups the number of predicted species is arbitrarily doubled. Most of these predictions are highly subjective. Molecular techniques such as environmental DNA barcoding have revealed a vast diversity of undescribed protists that accounts for the majority of eukaryotic sequences or operational taxonomic units (OTUs), dwarfing those from plants, animals and fungi.[47] As such, it is considered that protists dominate eukaryotic diversity.[6]
| Protist phylogeny |
| One possible topology for the eukaryotic tree of life.[49][2][50] Excavate groups shown in green. 1Includes land plants. 2Includes animals and fungi. |
The evolutionary relationships of protists have been explained through molecular phylogenetics, the sequencing of entire genomes and transcriptomes, and electron microscopy studies of the flagellar apparatus and cytoskeleton. New major lineages of protists and novel biodiversity continue to be discovered, resulting in dramatic changes to the eukaryotic tree of life. The newest classification systems of eukaryotes do not recognize the formal taxonomic ranks (kingdom, phylum, class, order...) and instead only recognize clades of related organisms, making the classification more stable in the long term and easier to update. In this new cladistic scheme, the protists are divided into various branches informally named supergroups. Most photosynthetic eukaryotes fall under the Diaphoretickes clade, which contains the supergroups Archaeplastida (which includes plants) and TSAR (including Telonemia, Stramenopiles, Alveolata and Rhizaria), as well as the phyla Cryptista and Haptista.[13] The animals and fungi fall into the Amorphea supergroup, which contains the phylum Amoebozoa and several other protist lineages. Various groups of eukaryotes with primitive cell architecture are collectively known as the Excavata.[1]
Excavata
[edit]Excavata is a group that encompasses diverse protists, mostly flagellates, ranging from aerobic and anaerobic predators to phototrophs and heterotrophs.[51]: 597 The common name 'excavate' refers to the common characteristic of a ventral groove in the cell used for suspension feeding, which is considered to be an ancestral trait present in the last eukaryotic common ancestor.[52] The Excavata is composed of three clades: Discoba, Metamonada and Malawimonadida, each including 'typical excavates' that are free-living phagotrophic flagellates with the characteristic ventral groove.[53] According to most phylogenetic analyses, this group is paraphyletic, with some analyses placing the root of the eukaryote tree within Metamonada.[54]
Discoba includes three major groups: Jakobida, Euglenozoa and Percolozoa.[b] Jakobida are a small group (~20 species) of free-living heterotrophic flagellates, with two cilia, that primarily eat bacteria through suspension feeding; most are aquatic aerobes, with some anaerobic species, found in marine, brackish or fresh water.[57] They are best known for their bacterial-like mitochondrial genomes.[13] Euglenozoa is a rich (>2,000 species)[58] group of flagellates with very different lifestyles, including: the free-living heterotrophic (both osmo- and phagotrophic)[51] and photosynthetic euglenids (e.g., the euglenophytes, with chloroplasts originated from green algae); the free-living and parasitic kinetoplastids (such as the trypanosomes); the deep-sea anaerobic symbiontids; and the elusive diplonemids.[59] Percolozoa[b] (~150 species) are a collection of amoebae, flagellates and amoeboflagellates with complex life cycles, among which are some slime molds (acrasids).[13][55] The two clades Euglenozoa and Percolozoa are sister taxa, united under the name Discicristata, in reference to their mitochondrial cristae shaped like discs.[56] The species Tsukubamonas globosa is a free-living flagellate whose precise position within Discoba is not yet settled, but is probably more closely related to Discicristata than to Jakobida.[57]
The metamonads (Metamonada) are a phylum of completely anaerobic or microaerophilic protozoa, primarily flagellates. Some are gut symbionts of animals such as termites, others are free-living, and others are parasitic. They include three main clades: Fornicata, Parabasalia and Preaxostyla.[13] Fornicata (>140 species)[60] encompasses the diplomonads, with two nuclei (e.g., Giardia, genus of well-known parasites of humans), and several smaller groups of free-living, commensal and parasitic protists (e.g., Carpediemonas, retortamonads).[13] Parabasalia (>460 species)[60] is a varied group of anaerobic, mostly endobiotic organisms, ranging from small parasites (like Trichomonas vaginalis, another human pathogen) to giant intestinal symbionts with numerous flagella and nuclei found in wood-eating termites and cockroaches.[13] Preaxostyla (~140 species) includes the anaerobic and endobiotic oxymonads, with modified mitochondria, and two genera of free-living microaerophilic bacterivorous flagellates Trimastix and Paratrimastix, with typical excavate morphology.[61] Two genera of anaerobic flagellates of recent description and unique cell architecture, Barthelona and Skoliomonas, are closely related to the Fornicata.[62]
The malawimonads (Malawimonadida) are a small group (3 species) of freshwater or marine suspension-feeding bacterivorous flagellates[63] with typical excavate appearance, closely resembling Jakobida and some metamonads but not phylogenetically close to either in most analyses.[13]
Diaphoretickes
[edit]Diaphoretickes includes nearly all photosynthetic eukaryotes. Within this clade, the TSAR supergroup gathers a colossal diversity of protists. The most basal branching member of the TSAR clade is Telonemia, a small (7 species) phylum of obscure phagotrophic predatory flagellates, found in marine and freshwater environments. They share some cellular similarities with the remaining three clades: Rhizaria, Alveolata and Stramenopiles, collectively known as the SAR supergroup.[64] Another highly diverse clade within Diaphoretickes is Archaeplastida, which houses land plants and a variety of algae. In addition, two smaller groups, Haptista and Cryptista, also belong to Diaphoretickes.[1]
Stramenopiles
[edit]The Stramenopiles, also known as Heterokonta, are characterized by the presence of two cilia, one of which bears many short, straw-like hairs (mastigonemes). They include one clade of phototrophs and numerous clades of heterotrophs, present in virtually all habitats. Stramenopiles include two usually well-supported clades, Bigyra and Gyrista, although the monophyly of Bigyra is being questioned.[65] Branching outside both Bigyra and Gyrista is a single species of enigmatic heterotrophic flagellates, Platysulcus tardus.[65] Much of the diversity of heterotrophic stramenopiles is still uncharacterized, known almost entirely from lineages of genetic sequences known as MASTs (MArine STramenopiles),[65] of which only a few species have been described.[66][67]
The phylum Gyrista includes the photosynthetic Ochrophyta or Heterokontophyta (>23,000 species),[58] which contain chloroplasts originated from a red alga. Among these are many lineages of algae that encompass a wide range of structures and morphologies. The three most diverse ochrophyte classes are: the diatoms, unicellular or colonial organisms encased in silica cell walls (frustules) that exhibit widely different shapes and ornamentations, responsible for a big portion of the oxygen produced worldwide, and comprising much of the marine phytoplankton;[13][68] the brown algae, filamentous or 'truly' multicellular (with differentiated tissues) macroalgae that constitute the basis of many temperate and cold marine ecosystems, such as kelp forests;[69] and the golden algae, unicellular or colonial flagellates that are mostly present in freshwater habitats.[70] Inside Gyrista, the sister clade to Ochrophyta are the predominantly osmotrophic and filamentous Pseudofungi (>1,200 species),[71] which include three distinct lineages: the parasitic oomycetes or water moulds (e.g., Phytophthora infestans, the agent behind the Irish Potato Famine), which encompass most of the pseudofungi species; the less diverse non-parasitic hyphochytrids that maintain a fungus-like lifestyle; and the bigyromonads, a group of bacterivorous or eukaryovorous phagotrophs.[65] A small group of heliozoan-like heterotrophic amoebae, Actinophryida, has an uncertain position, either within or as the sister taxon of Ochrophyta.[72]
The little studied phylum Bigyra is an assemblage of exclusively heterotrophic organisms, most of which are free-living. It includes the Labyrinthulomycetes, among which are single-celled amoeboid phagotrophs, mixotrophs, and fungus-like filamentous heterotrophs that create slime networks to move and absorb nutrients, as well as some parasites. Also included in Bigyra are the bicosoecids, phagotrophic flagellates that consume bacteria, and the closely related Placidozoa, which consists of several groups of heterotrophic flagellates (e.g., the deep-sea halophilic Placididea) as well as the intestinal commensals known as Opalinata (e.g., the human parasite Blastocystis, and the highly unusual opalinids, composed of giant cells with numerous nuclei and cilia, originally misclassified as ciliates).[65]
Alveolata
[edit]The alveolates (Alveolata) are characterized by the presence of cortical alveoli, cytoplasmic sacs underlying the cell membrane of unknown physiological function.[51]: 599 Among them are three of the most well-known groups of protists: apicomplexans, dinoflagellates and ciliates. The ciliates (Ciliophora) are a well-studied,[13] highly diverse (>8,000 species) group of mostly free-living microbes characterized by large cells covered in rows of cilia and containing two kinds of nuclei, micronucleus and macronucleus (e.g., Paramecium, a model organism).[73] Free-living ciliates are usually the top heterotrophs and predators in microbial food webs, feeding on bacteria and smaller eukaryotes, present in a variety of ecosystems, although a few species are kleptoplastic. Others are parasitic of numerous animals.[74] Ciliates have a basal position in the evolution of alveolates, together with a few species of heterotrophic flagellates with two cilia collectively known as colponemids.[75]
The remaining alveolates are grouped under the clade Myzozoa, whose common ancestor acquired chloroplasts through a secondary endosymbiosis from a red alga.[76] One branch of Myzozoa contains the apicomplexans and their closest relatives, a small clade of flagellates known as Chrompodellida where phototrophic and heterotrophic flagellates, called chromerids and colpodellids respectively, are evolutionarily intermingled.[76] In contrast, the apicomplexans (Apicomplexa) are a large (>6,000 species) and highly specialized group of obligate parasites who have all secondarily lost their photosynthetic ability (e.g., Plasmodium falciparum, cause of malaria). Their adult stages absorb nutrients from the host through the cell membrane, and they reproduce between hosts via sporozoites, which exhibit an organelle complex (the apicoplast) evolved from non-photosynthetic chloroplasts.[77][51]: 600
The other branch of Myzozoa contains the dinoflagellates and their closest relatives, the perkinsids (Perkinsozoa), a small group (26 species) of aquatic intracellular parasites which have lost their photosynthetic ability similarly to apicomplexans.[76] They reproduce through flagellated spores that infect dinoflagellates, molluscs and fish.[78] In contrast, the dinoflagellates (Dinoflagellata) are a highly diversified (~4,500 species)[79] group of aquatic algae that have mostly retained their chloroplasts, although many lineages have lost their own and instead either live as heterotrophs or reacquire new chloroplasts from other sources, including tertiary endosymbiosis and kleptoplasty.[80] Most dinoflagellates are free-living and compose an important portion of phytoplankton, as well as a major cause of harmful algal blooms due to their toxicity; some live as symbionts of corals, allowing the creation of coral reefs. Dinoflagellates exhibit a diversity of cellular structures, such as complex eyelike ocelli, specialized vacuoles, bioluminescent organelles, and a wall surrounding the cell known as the theca.[79]
Rhizaria
[edit]Rhizaria is a lineage of morphologically diverse organisms, comprising mostly heterotrophic amoebae, flagellates and amoeboflagellates. It was the last supergroup to be discovered, because it lacks any defining characteristic and was described exclusively through molecular phylogenetics. The most familiar rhizarians are Foraminifera and Radiolaria, groups of large and abundant marine amoebae, many of them macroscopic. Much of the rhizarian diversity lies within the phylum Cercozoa, filled with free-living flagellates which usually have pseudopodia, as well as Phaeodaria, a group previously considered radiolarian. Other groups comprise various amoebae like Vampyrellida or are important parasites like Phytomyxea, Paramyxida or Haplosporida.[13]
Haptista and Cryptista
[edit]Haptista and Cryptista are two similar protist phyla previously thought to be closely related, and collectively known as Hacrobia.[81] However, the monophyly of Hacrobia was disproven, with molecular analyses placing Cryptista next to Archaeplastida, forming the hypothesized "CAM" clade, and Haptista next to the TSAR clade.[82][50]
Haptista — includes the Haptophyta algae and the heterotrophic Centrohelida, which are "heliozoan"-type amoebae.[13]
Cryptista — closely related to Archaeplastida, it includes the Cryptophyta algae, with a plastid of red algal origin, and two obscure relatives with two flagella, katablepharids and Palpitomonas.[13]
Archaeplastida
[edit]The Archaeplastida or Plantae[c] consists of groups that have evolved from a common photosynthetic ancestor that obtained chloroplasts directly through a single event of endosymbiosis with a cyanobacterium. These are:
- Glaucophyta (26 species), unicellular algae found in freshwater and terrestrial environments.[83]
- Picozoa (1 species), non-photosynthetic predators.[84]
- Rhodophyta (5,000–6,000 species), mostly multicellular marine algae that lost chlorophyll and only harvest light energy through phycobiliproteins.[83]
- Rhodelphidia (2 species), predators with non-photosynthetic plastid.[84]
- Viridiplantae or Chloroplastida, containing both green algae and land plants. The green algae comprise many lineages of varying diversity, such as Chlorophyta (7,000), Prasinodermophyta (10), Zygnematophyceae (4,000), Charophyceae (877), Klebsormidiophyceae (48) or Coleochaetophyceae (36).[13]
Amorphea
[edit]Amoebozoa
[edit]The phylum Amoebozoa (2,400 species) is a large group of heterotrophic protists, mostly amoebae. Many lineages are slime molds that produce spore-releasing fruiting bodies, such as Myxogastria, Dictyostelia and Protosporangiida, and are often studied by mycologists. Within the non-fruiting amoebae, the Tubulinea contain many naked amoebae (such as Amoeba itself) and a well-studied order of testate amoebae known as Arcellinida. Other non-fruiting amoebozoans are Variosea, Discosea and Archamoebae.[13]
Obazoa
[edit]Obazoa includes the two kingdoms Metazoa (animals) and Fungi,[d] and their closest protist relatives inside a clade known as Opisthokonta. The opisthokont protists are Nucleariida, Ichthyosporea, Pluriformea, Filasterea, Choanoflagellata and the elusive Tunicaraptor (1 species).[87] They are flagellated or amoeboid heterotrophs of vital importance in the search for the genes that allow animal multicellularity. Sister groups to Opisthokonta are Apusomonadida (28 species)[88] and Breviatea (4 species).[13]
Orphan groups
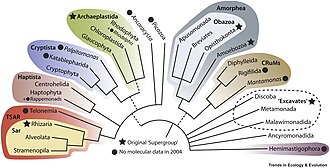
[edit]Many smaller lineages do not belong to any of these supergroups, and are usually poorly known groups with limited data, often referred to as 'orphan groups'. Some, such as the CRuMs clade, Malawimonadida and Ancyromonadida, appear to be related to Amorphea.[13] Others, like Hemimastigophora (10 species)[89] and Provora (7 species), appear to be related to or within Diaphoretickes, a clade that unites SAR, Archaeplastida, Haptista and Cryptista.[2] A mysterious protist species, Meteora sporadica, is more closely related to the latter two of these orphan groups.[50]
Ecology
[edit]Protists make up a large portion of the biomass in both marine and terrestrial ecosystems. It has been estimated that protists account for 4 gigatons (Gt) of biomass in the entire planet Earth. This amount is smaller than 1% of all biomass, but is still double the amount estimated for all animals (2 Gt). Together, protists, animals, archaea (7 Gt) and fungi (12 Gt) account for less than 10% of the total biomass of the planet, because plants (450 Gt) and bacteria (70 Gt) are the remaining 80% and 15% respectively.[90]
Habitats
[edit]Protists are highly abundant and diverse in all types of ecosystems, especially free-living (i.e. non-parasitic) groups. An unexpectedly enormous, taxonomically undescribed diversity of eukaryotic microbes is detected everywhere in the form of environmental DNA or RNA. The richest protist communities appear in soil, followed by ocean and freshwater habitats.[91]
Phagotrophic protists (consumers) are the most diverse functional group in all ecosystems, with three main taxonomical groups of phagotrophs: Rhizaria (mainly Cercozoa in freshwater and soil habitats, and Radiolaria in oceans), ciliates (most abundant in freshwater and second most abundant in soil) and non-photosynthetic stramenopiles (third most represented overall, higher in soil than in oceans). Phototrophic protists (producers) appear in lower proportions, probably constrained by intense predation. They exist in similar abundance in both oceans and soil. They are mostly dinophytes in oceans, chrysophytes in freshwater, and Archaeplastida in soil.[91]
Marine
[edit]
Marine protists are highly diverse, have a fundamental impact on biogeochemical cycles (particularly, the carbon cycle)[92] and are at the base of the marine trophic networks as part of the plankton.[93]
Phototrophic marine protists located in the photic zone as phytoplankton are vital primary producers in the oceanic systems. They fix as much carbon as all terrestrial plants together.[91] The smallest fractions, the picoplankton (<2 μm) and nanoplankton (2–20 μm), are dominated by several different algae (prymnesiophytes, pelagophytes, prasinophytes); fractions larger than 5 μm are instead dominated by diatoms and dinoflagellates. The heterotrophic fraction of marine picoplankton encompasses primarily early-branching stramenopiles (e.g. bicosoecids and labyrinthulomycetes), as well as alveolates, ciliates and radiolarians; protists of lower frequency include cercozoans and cryptophytes.[94]
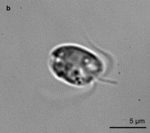
Mixotrophic marine protists, while not very researched, are present abundantly and ubiquitously in the global oceans, on a wide range of marine habitats. In metabarcoding analyses, they constitute more than 12% of the environmental sequences. They are an important and underestimated source of carbon in eutrophic and oligotrophic habitats.[93] Their abundance varies seasonally.[95] Planktonic protists are classified into various functional groups or 'mixotypes' that present different biogeographies:
- Constitutive mixotrophs, also called 'phytoplankton that eat', have the innate ability to photosynthesize. They have diverse feeding behaviors: some require phototrophy, others phagotrophy, and others are obligate mixotrophs.[93] They are responsible for harmful algal blooms. They dominate the eukaryotic microbial biomass in the photic zone, in eutrophic and oligotrophic waters across all climate zones, even in non-bloom conditions. They account for significant, often dominant predation of bacteria.[96]
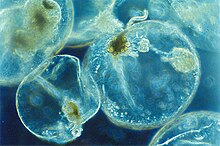
- Non-constitutive mixotrophs acquire the ability to photosynthesize by stealing chloroplasts from their prey. They can be divided into two: generalists, which can use chloroplasts stolen from a variety of prey (e.g. oligotrich ciliates), or specialists, which have developed the need to only acquire chloroplasts from a few specific prey. The specialists are further divided into two: plastidic, those which contain differentiated plastids (e.g. Mesodinium, Dinophysis), and endosymbiotic, those which contain endosymbionts (e.g. mixotrophic Rhizaria such as Foraminifera and Radiolaria, dinoflagellates like Noctiluca).[96] Both plastidic and generalist non-constitutive mixotrophs have similar biogeographies and low abundance, mostly found in eutrophic coastal waters. Generalist ciliates can account for up to 50% of ciliate communities in the photic zone. The endosymbiotic mixotrophs are the most abundant non-constitutive type.[93]
Freshwater
[edit]Freshwater planktonic protist communities are characterized by a higher "beta diversity" (i.e. highly heterogeneous between samples) than soil and marine plankton. The high diversity can be a result of the hydrological dynamic of recruiting organisms from different habitats through extreme floods.[97] The main freshwater producers (chrysophytes, cryptophytes and dinophytes) behave alternatively as consumers (mixotrophs). At the same time, strict consumers (non-photosynthetic) are less abundant in freshwater, implying that the consumer role is partly taken by these mixotrophs.[91]
Soil
[edit]Soil protist communities are ecologically the richest. This may be due to the complex and highly dynamic distribution of water in the sediment, which creates extremely heterogenous environmental conditions. The constantly changing environment promotes the activity of only one part of the community at a time, while the rest remains inactive; this phenomenon promotes high microbial diversity in prokaryotes as well as protists. Only a small fraction of the detected diversity of soil-dwelling protists has been described (8.1% as of 2017).[91] Soil protists are also morphologically and functionally diverse, with four major categories:[98]


- Photoautotrophic soil protists, or algae, are as abundant as their marine counterparts. Given the importance of marine algae, soil algae may provide a larger contribution to the global carbon cycle than previously thought, but the magnitude of their carbon fixation has yet to be quantified.[91] Most soil algae belong to the supergroups Stramenopiles (diatoms, Xanthophyceae and Eustigmatophyceae) and Archaeplastida (Chlorophyceae and Trebouxiophyceae). There is also the presence of environmental DNA from dinoflagellates and haptophytes in soil, but no living forms have been seen.[98]
- Fungus-like protists are present abundantly in soil. Most environmental sequences belong to the Oomycetes (Stramenopiles), an osmotrophic and saprotrophic group that contains free-living and parasitic species of other protists, fungi, plants and animals. Another important group in soil are slime molds (found in Amoebozoa, Opisthokonta, Rhizaria and Heterolobosea), which reproduce by forming fruiting bodies known as sporocarps (originated from a single cell) and sorocarps (from aggregations of cells).[98]
- Phagotrophic protists are abundant and essential in soil ecosystems. As bacterial grazers, they have a significant role in the foodweb: they excrete nitrogen in the form of NH3, making it available to plants and other microbes.[99] Many soil protists are also mycophagous, and facultative (i.e. non-obligate) mycophagy is a widespread evolutionary feeding mode among soil protozoa.[100] Amoeboflagellates like the glissomonads and cercomonads (in Rhizaria) are among the most abundant soil protists: they possess both flagella and pseudopodia, a morphological variability well suited for foraging between soil particles. Testate amoebae (e.g. arcellinids and euglyphids) have shells that protect against desiccation and predation, and their contribution to the silica cycle through the biomineralization of shells is as important as that of forest trees.[98]
- Parasitic soil protists (in Apicomplexa) are diverse, ubiquitous and have an important role as parasites of soil-dwelling invertebrate animals. In Neotropical forests, environmental DNA from the apicomplexan gregarines dominates protist diversity.[98]
Parasitic
[edit]
Parasitic protists represent around 15–20% of all environmental DNA in marine and soil systems, but only around 5% in freshwater systems, where chytrid fungi likely fill that ecological niche. In oceanic systems, parasitoids (i.e. those which kill their hosts, e.g. Syndiniales) are more abundant. In soil ecosystems, true parasites (i.e. those which do not kill their hosts) are primarily animal-hosted Apicomplexa (Alveolata) and plant-hosted oomycetes (Stramenopiles) and plasmodiophorids (Rhizaria). In freshwater ecosystems, parasitoids are mainly Perkinsea and Syndiniales (Alveolata), as well as the fungal Chytridiomycota. True parasites in freshwater are mostly oomycetes, Apicomplexa and Ichthyosporea.[91]
Some protists are significant parasites of animals (e.g.; five species of the parasitic genus Plasmodium cause malaria in humans and many others cause similar diseases in other vertebrates), plants[101][102] (the oomycete Phytophthora infestans causes late blight in potatoes)[103] or even of other protists.[104][105]
Around 100 protist species can infect humans.[98] Two papers from 2013 have proposed virotherapy, the use of viruses to treat infections caused by protozoa.[106][107]
Researchers from the Agricultural Research Service are taking advantage of protists as pathogens to control red imported fire ant (Solenopsis invicta) populations in Argentina. Spore-producing protists such as Kneallhazia solenopsae (recognized as a sister clade or the closest relative to the fungus kingdom now)[108] can reduce red fire ant populations by 53–100%.[109] Researchers have also been able to infect phorid fly parasitoids of the ant with the protist without harming the flies. This turns the flies into a vector that can spread the pathogenic protist between red fire ant colonies.[110]
History of protist classification
[edit]Early classifications
[edit]
From the start of the 18th century, the popular term "infusion animals" (later infusoria) referred to protists, bacteria and small invertebrate animals. In the mid-18th century, while Swedish scientist Carl von Linnaeus largely ignored the protists,[e] his Danish contemporary Otto Friedrich Müller was the first to introduce protists to the binomial nomenclature system.[111][112]
In the early 19th century, German naturalist Georg August Goldfuss introduced Protozoa (meaning 'early animals') as a class within Kingdom Animalia,[113] to refer to four very different groups: infusoria (ciliates), corals, phytozoa (such as Cryptomonas) and jellyfish. Later, in 1845, Carl Theodor von Siebold was the first to establish Protozoa as a phylum of exclusively unicellular animals consisting of two classes: Infusoria (ciliates) and Rhizopoda (amoebae, foraminifera).[114] Other scientists did not consider all of them part of the animal kingdom, and by the middle of the century they were regarded within the groupings of Protozoa (early animals), Protophyta (early plants), Phytozoa (animal-like plants) and Bacteria (mostly considered plants). Microscopic organisms were increasingly constrained in the plant/animal dichotomy. In 1858, the palaeontolgist Richard Owen was the first to define Protozoa as a separate kingdom of eukaryotic organisms, with "nucleated cells" and the "common organic characters" of plants and animals, although he also included sponges within protozoa.[24]
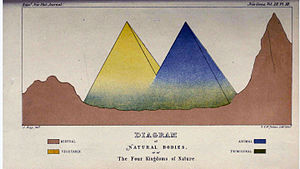
In 1860, British naturalist John Hogg proposed Protoctista (meaning 'first-created beings') as the name for a fourth kingdom of nature (the other kingdoms being Linnaeus' plant, animal and mineral) which comprised all the lower, primitive organisms, including protophyta, protozoa and sponges, at the merging bases of the plant and animal kingdoms.[115][24]
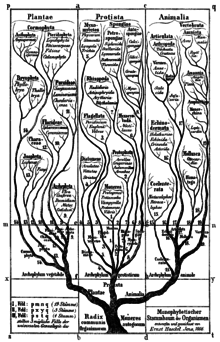
In 1866 the 'father of protistology', German scientist Ernst Haeckel, addressed the problem of classifying all these organisms as a mixture of animal and vegetable characters, and proposed Protistenreich[116] (Kingdom Protista) as the third kingdom of life, comprising primitive forms that were "neither animals nor plants". He grouped both bacteria[117] and eukaryotes, both unicellular and multicellular organisms, as Protista. He retained the Infusoria in the animal kingdom, until German zoologist Otto Bütschli demonstrated that they were unicellular.[118][119] At first, he included sponges and fungi, but in later publications he explicitly restricted Protista to predominantly unicellular organisms or colonies incapable of forming tissues. He clearly separated Protista from true animals on the basis that the defining character of protists was the absence of sexual reproduction, while the defining character of animals was the blastula stage of animal development. He also returned the terms protozoa and protophyta as subkingdoms of Protista.[24]
End of the animal-plant dichotomy
[edit]Bütschli considered the kingdom to be too polyphyletic and rejected the inclusion of bacteria. He fragmented the kingdom into protozoa (only nucleated, unicellular animal-like organisms), while bacteria and the protophyta were a separate grouping. This strengthened the old dichotomy of protozoa/protophyta from German scientist Carl Theodor von Siebold, and the German naturalists asserted this view over the worldwide scientific community by the turn of the century. However, British biologist C. Clifford Dobell in 1911 brought attention to the fact that protists functioned very differently compared to the animal and vegetable cellular organization, and gave importance to Protista as a group with a different organization that he called "acellularity", shifting away from the dogma of German cell theory. He coined the term protistology and solidified it as a branch of study independent from zoology and botany.[24]
In 1938, American biologist Herbert Copeland resurrected Hogg's label, arguing that Haeckel's term Protista included anucleated microbes such as bacteria, which the term Protoctista (meaning "first established beings") did not. Under his four-kingdom classification (Monera, Protoctista, Plantae, Animalia), the protists and bacteria were finally split apart, recognizing the difference between anucleate (prokaryotic) and nucleate (eukaryotic) organisms. To firmly separate protists from plants, he followed Haeckel's blastular definition of true animals, and proposed defining true plants as those with chlorophyll a and b, carotene, xanthophyll and production of starch. He also was the first to recognize that the unicellular/multicellular dichotomy was invalid. Still, he kept fungi within Protoctista, together with red algae, brown algae and protozoans.[24][120] This classification was the basis for Whittaker's later definition of Fungi, Animalia, Plantae and Protista as the four kingdoms of life.[121]
In the popular five-kingdom scheme published by American plant ecologist Robert Whittaker in 1969, Protista was defined as eukaryotic "organisms which are unicellular or unicellular-colonial and which form no tissues". Just as the prokaryotic/eukaryotic division was becoming mainstream, Whittaker, after a decade from Copeland's system,[121] recognized the fundamental division of life between the prokaryotic Monera and the eukaryotic kingdoms: Animalia (ingestion), Plantae (photosynthesis), Fungi (absorption) and the remaining Protista.[122][123][24]
In the five-kingdom system of American evolutionary biologist Lynn Margulis, the term "protist" was reserved for microscopic organisms, while the more inclusive kingdom Protoctista (or protoctists) included certain large multicellular eukaryotes, such as kelp, red algae, and slime molds.[124] Some use the term protist interchangeably with Margulis' protoctist, to encompass both single-celled and multicellular eukaryotes, including those that form specialized tissues but do not fit into any of the other traditional kingdoms.[125]
Advances in electron microscopy and molecular phylogenetics
[edit]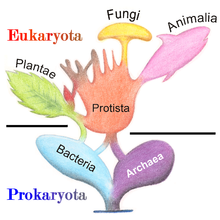
The five-kingdom model remained the accepted classification until the development of molecular phylogenetics in the late 20th century, when it became apparent that protists are a paraphyletic group from which animals, fungi and plants evolved, and the three-domain system (Bacteria, Archaea, Eukarya) became prevalent.[126] Today, protists are not treated as a formal taxon, but the term is commonly used for convenience in two ways:[5]
- Phylogenetic definition: protists are a paraphyletic group.[127] A protist is any eukaryote that is not an animal, land plant or fungus,[128] thus excluding many unicellular groups like the fungal Microsporidia, Chytridiomycetes and yeasts, and the non-unicellular Myxozoan animals included in Protista in the past.[129]
- Functional definition: protists are essentially those eukaryotes that are never multicellular,[5] that either exist as independent cells, or if they occur in colonies, do not show differentiation into tissues.[130] While in popular usage, this definition excludes the variety of non-colonial multicellularity types that protists exhibit, such as aggregative (e.g. choanoflagellates) or complex multicellularity (e.g. brown algae).[131]
There is, however, one classification of protists based on traditional ranks that lasted until the 21st century. The British protozoologist Thomas Cavalier-Smith, since 1998, developed a six-kingdom model:[f] Bacteria, Animalia, Plantae, Fungi, Protozoa and Chromista.[10][132] In his context, paraphyletic groups take preference over clades:[10] both protist kingdoms Protozoa and Chromista contain paraphyletic phyla such as Apusozoa, Eolouka or Opisthosporidia. Additionally, red and green algae are considered true plants, while the fungal groups Microsporidia, Rozellida and Aphelida are considered protozoans under the phylum Opisthosporidia. This scheme endured until 2021, the year of his last publication.[56]
Evolution and fossil record
[edit]Mesoproterozoic
[edit]By definition, all eukaryotes before the existence of plants, animals and fungi are considered protists. For that reason, this section contains information about the deep ancestry of all eukaryotes.
All living eukaryotes, including protists, evolved from the last eukaryotic common ancestor (LECA). Descendants of this ancestor are known as "crown-group" or "modern" eukaryotes. Molecular clocks suggest that LECA originated between 1200 and more than 1800 million years ago (Ma). Based on all molecular predictions, modern eukaryotes reached morphological and ecological diversity before 1000 Ma in the form of multicellular algae capable of sexual reproduction, and unicellular protists capable of phagocytosis and locomotion. However, the fossil record of modern eukaryotes is very scarce around this period, which contradicts the predicted diversity.[133]
Instead, the fossil record of this period contains "stem-group eukaryotes". These fossils cannot be assigned to any known crown group, so they probably belong to extinct lineages that originated before LECA. They appear continuously throughout the Mesoproterozoic fossil record (1650–1000 Ma). They present defining eukaryote characteristics such as complex cell wall ornamentation and cell membrane protrusions, which require a flexible endomembrane system. However, they had a major distinction from crown eukaryores: the composition of their cell membrane. Unlike crown eukaryotes, which produce "crown sterols" for their cell membranes (e.g. cholesterol and ergosterol), stem eukaryotes produced "protosterols", which appear earlier in the biosynthetic pathway.[133]
Crown sterols, while metabolically more expensive, may have granted several evolutionary advantages for LECA's descendants. Specific unsaturation patterns in crown sterols protect against osmotic shock during desiccation and rehydration cycles. Crown sterols can also receive ethyl groups, thus enhancing cohesion between lipids and adapting cells against extreme cold and heat. Moreover, the additional steps in the biosynthetic pathway allow cells to regulate the proportion of different sterols in their membranes, in turn allowing for a wider habitable temperature range and unique mechanisms such as asymmetric cell division or membrane repair under exposure to UV light. A more speculative role of these sterols is their protection against the Proterozoic changing oxygen levels. It is theorized that all of these sterol-based mechanisms allowed LECA's descendants to live as extremophiles of their time, diversifying into ecological niches that experienced cycles of desiccation and rehydration, daily extremes of high and low temperatures, and elevated UV radiation (such as mudflats, rivers, agitated shorelines and subaerial soil).[133]
In contrast, the named mechanisms were absent in stem-group eukaryotes, as they were only capable of producing protosterols. Instead, these protosterol-based life forms occupied open marine waters. They were facultative anaerobes that thrived in Mesoproterozoic waters, which at the time were low on oxygen. Eventually, during the Tonian period (Neoproterozoic era), oxygen levels increased and the crown eukaryotes were able to expand to open marine environments thanks to their preference for more oxygenated habitats. Stem eukaryotes may have been driven to extinction as a result of this competition. Additionally, their protosterol membranes may have posed a disadvantage during the cold of the Cryogenian "Snowball Earth" glaciations and the extreme global heat that came afterwards.[133]
Neoproterozoic
[edit]Modern eukaryotes began to appear abundantly in the Tonian period (1000–720 Ma), fueled by the proliferation of red algae. The oldest fossils assigned to modern eukaryotes belong to two photosynthetic protists: the multicellular red alga Bangiomorpha (from 1050 Ma), and the chlorophyte green alga Proterocladus (from 1000 Ma).[133] Abundant fossils of heterotrophic protists appear later, around 900 Ma, with the emergence of fungi.[133] For example, the oldest fossils of Amoebozoa are vase-shaped microfossils resembling modern testate amoebae, found in 800 million-year-old rocks.[134][135] Radiolarian shells are found abundantly in the fossil record after the Cambrian period (~500 Ma), but more recent paleontological studies are beginning to interpret some Precambrian fossils as the earliest evidence of radiolarians.[136][137][138]
See also
[edit]Footnotes
[edit]- ^ The terms 'cilium' and 'eukaryotic flagellum' are interchangeable from a biological perspective. However, their usage depends on the author: some prefer to reserve cilia for shorter appendages and flagella for longer ones, while others prefer cilia for eukaryotes and flagella for prokaryotes. The term 'undulipodium' was proposed to unify the two concepts, as it refers specifically to the homologous microtubular structure found in both, but not found in prokaryotic flagella.[20][21][22]
- ^ a b The phylum Percolozoa is usually better known as Heterolobosea.[1][55] However, in the strictest sense, Heterolobosea refers only to a class within this phylum, containing the orders Acrasida and Schizopyrenida. The name Percolozoa encompasses these and other related single-celled protists, not just the 'true' heteroloboseans.[56]
- ^ According to some classifications,[10] all of Archaeplastida is treated as Kingdom Plantae, but others consider the algae (or non-terrestrial "plants") to be protists.[13]
- ^ Under traditional classifications, the groups Microsporidia, Aphelida and Rozellida are considered to be protists, commonly grouped by the name Opisthosporidia and treated as the immediate relative of Eumycota or true fungi.[56] However, many researchers currently accept those three groups as part of a larger Kingdom Fungi.[1][85][86]
- ^ Carl von Linnaeus did not mention a single protist genus until the tenth edition of Systema Naturae of 1758, where Volvox was recorded.[111]
- ^ In 2015, Cavalier-Smith's initial six-kingdom model was revised into a seven-kingdom model after the inclusion of Archaea.[132]
References
[edit]- ^ a b c d e f Adl, Sina M.; Bass, David; Lane, Christopher E.; Lukeš, Julius; Schoch, Conrad L.; Smirnov, Alexey; et al. (2019). "Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes". Journal of Eukaryotic Microbiology. 66 (1): 4–119. doi:10.1111/JEU.12691. PMC 6492006. PMID 30257078.
- ^ a b c Tikhonenkov DV, Mikhailov KV, Gawryluk RMR, et al. (2022). "Microbial predators form a new supergroup of eukaryotes". Nature. 612 (7941): 714–719. Bibcode:2022Natur.612..714T. doi:10.1038/s41586-022-05511-5. PMID 36477531. S2CID 254436650.
- ^ Simonite T (November 2005). "Protists push animals aside in rule revamp". Nature. 438 (7064): 8–9. Bibcode:2005Natur.438....8S. doi:10.1038/438008b. PMID 16267517.
- ^ Harper D, Benton, Michael (2009). Introduction to Paleobiology and the Fossil Record. Wiley-Blackwell. p. 207. ISBN 978-1-4051-4157-4.
- ^ a b c O'Malley MA, Simpson AG, Roger AJ (2012). "The other eukaryotes in light of evolutionary protistology". Biology & Philosophy. 28 (2): 299–330. doi:10.1007/s10539-012-9354-y. S2CID 85406712.
- ^ a b Burki, Fabien; Sandin, Miguel M.; Jamy, Mahwash (2021). "Diversity and ecology of protists revealed by metabarcoding". Current Biology. 31 (19): R1267–R1280. Bibcode:2021CBio...31R1267B. doi:10.1016/j.cub.2021.07.066. PMID 34637739. S2CID 238588753.
- ^ a b Levine ND, Corliss JO, Cox FEG, Deroux G, Grain J, Honigberg BM, et al. (1980). "A newly revised classification of the Protozoa". Journal of Protozoology. 27 (1): 37–58. doi:10.1111/j.1550-7408.1980.tb04228.x. PMID 6989987.
- ^ Weiss, Louis M. (2001). "Microsporidia: emerging pathogenic protists". Acta Tropica. 78 (2): 89–102. doi:10.1016/S0001-706X(00)00178-9. PMID 11230819."
- ^ Karpov, Sergey A.; Mamkaeva, Maria A.; Aleoshin, Vladimir V.; Nassonova, Elena; Lilje, Osu; Gleason, Frank H. (2014). "Morphology, phylogeny, and ecology of the aphelids (Aphelidea, Opisthokonta) and proposal for the new superphylum Opisthosporidia". Frontiers in Microbiology. 5: 112. doi:10.3389/fmicb.2014.00112. PMC 3975115. PMID 24734027.
- ^ a b c d Cavalier-Smith T (August 1998). "A revised six-kingdom system of life". Biological Reviews of the Cambridge Philosophical Society. 73 (3): 203–266. doi:10.1111/j.1469-185X.1998.tb00030.x. PMID 9809012.
- ^ Corliss, J.O. (1995). "The ambiregnal protists and the codes of nomenclature: a brief review of the problem and of proposed solutions". Bulletin of Zoological Nomenclature. 52: 11–17. doi:10.5962/bhl.part.6717.
- ^ Richard Barnes; Stephen Kent (2001). The Invertebrates: A Synthesis. Wiley-Blackwell. p. 41. ISBN 978-0-632-04761-1.
- ^ a b c d e f g h i j k l m n o p q r s t Simpson, Alastair G. B.; Slamovits, Claudio H.; Archibald, John M. (2017). "Protist Diversity and Eukaryote Phylogeny". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 1–22. doi:10.1007/978-3-319-28149-0_45. ISBN 978-3-319-28147-6.
- ^ De Bruyn, P. P. H. (March 1947). "Theories of amoeboid movement". The Quarterly Review of Biology. 22 (1). The University of Chicago Press: 1–24. doi:10.1086/395577. JSTOR 2813332. PMID 20287832.
- ^ Brown MW, Kolisko M, Silberman JD, Roger AJ (2012). "Aggregative Multicellularity Evolved Independently in the Eukaryotic Supergroup Rhizaria". Current Biology. 22 (12): 1123–1127. Bibcode:2012CBio...22.1123B. doi:10.1016/j.cub.2012.04.021. PMID 22608512. S2CID 17510471.
- ^ Pawlowski, Jan (2008). "The twilight of Sarcodina: a molecular perspective on the polyphyletic origin of amoeboid protists" (PDF). Protistology. 5 (4): 281–302.
- ^ Pawlowski, Jan; Burki, Fabien (2009). "Untangling the Phylogeny of Amoeboid Protists". The Journal of Eukaryotic Microbiology. 56 (1): 16–25. doi:10.1111/j.1550-7408.2008.00379.x. PMID 19335771.
- ^ Matz, Mikhail V.; Frank, Tamara M.; Marshall, N. Justin; Widder, Edith A.; Johnsen, Sönke (2008). "Giant deep-sea protist produces bilaterian-like traces". Current Biology. 18 (23): 1849–1854. Bibcode:2008CBio...18.1849M. doi:10.1016/j.cub.2008.10.028. PMID 19026540.
- ^ Levin, Lisa A.; Rouse, Greg W. (2019). "Giant protists (xenophyophores) function as fish nurseries". Ecology. 101 (4): e02933. doi:10.1002/ecy.2933. PMC 7341444. PMID 31742677.
- ^ "Lynn Margulis replies". BioScience. 36 (5): 293–294. 1986. doi:10.1093/bioscience/36.5.293-a.
- ^ Margulis, Lynn (1980). "Undulipodia, flagella and cilia". Biosystems. 12 (1–2): 105–108. Bibcode:1980BiSys..12..105M. doi:10.1016/0303-2647(80)90041-6. PMID 7378551.
- ^ Andersen, R. A.; Barr, D. J. S.; Lynn, D. H.; Melkonian, M.; Moestrup, Ø.; Sleigh, M. A. (1991). "Terminology and nomenclature of the cytoskeletal elements associated with the flagellar/ciliary apparatus in protists". Protoplasma. 164 (1–3): 1–8. doi:10.1007/BF01320809.
- ^ Thibaut Brunet; Marvin Albert; William Roman; Maxwell C Coyle; Danielle C Spitzer; Nicole King (15 January 2021). "A flagellate-to-amoeboid switch in the closest living relatives of animals". eLife. 10. doi:10.7554/ELIFE.61037. ISSN 2050-084X. PMC 7895527. PMID 33448265. Wikidata Q105870433.
- ^ a b c d e f g Scamardella JM (1999). "Not plants or animals: A brief history of the origin of Kingdoms Protozoa, Protista, and Protoctista". International Microbiology. 2 (4): 207–221. PMID 10943416.
- ^ Eliáš, Marek (2021). "Protist diversity: Novel groups enrich the algal tree of life". Current Biology. 31 (11): R714–R740. Bibcode:2021CBio...31.R733E. doi:10.1016/j.cub.2021.04.025.
- ^ Gleason, Frank H.; Lilje, Osu; Lange, Lene (2018). "What has happened to the "aquatic phycomycetes" (sensu Sparrow)? Part II: Shared properties of zoosporic true fungi and fungus-like microorganisms". Fungal Biology Reviews. 32 (2): 52–61. Bibcode:2018FunBR..32...52G. doi:10.1016/j.fbr.2017.09.003.
- ^ Neuhauser, Sigrid; Glockling, Sally L.; Leaño, Eduardo M.; Lilje, Osu; Marano, Agostina V.; Gleason, Frank H. (2012). "An introduction to fungus-like microorganisms". In Jones, E. B. Gareth; Pang, Ka-Lai (eds.). Marine fungi. Marine and Freshwater Botany. De Gruyter. pp. 137–152. doi:10.1515/9783110264067.137. ISBN 9783110264067.
- ^ Cavalier-Smith, Thomas (1993). "Kingdom Protozoa and its 18 phyla". Microbiological Reviews. 57 (4): 953–994. doi:10.1128/mr.57.4.953-994.1993. PMC 372943. PMID 8302218.
- ^ "Facts about malaria". www.ecdc.europa.eu. June 9, 2017.
- ^ Plattner H (2018). "Evolutionary cell biology of proteins from protists to humans and plants". J. Eukaryot. Microbiol. 65 (2): 255–289. doi:10.1111/jeu.12449. PMID 28719054. S2CID 206055044.
- ^ a b c d e Levandowsky, Michael (2012). "Physiological Adaptations of Protists". In Sperelakis, Nicholas (ed.). Cell Physiology Sourcebook: Essentials of Membrane Biophysics (Fourth ed.). Amsterdam; Boston: Elsevier/AP. pp. 874–890. ISBN 978-0-12-387738-3.
- ^ Hoppenrath, M; Bachvaroff, TR; Handy, SM; Delwiche, CF; Leander, BS (25 May 2009). "Molecular phylogeny of ocelloid-bearing dinoflagellates (Warnowiaceae) as inferred from SSU and LSU rDNA sequences". BMC Evolutionary Biology. 9 (1): 116. Bibcode:2009BMCEE...9..116H. doi:10.1186/1471-2148-9-116. PMC 2694157. PMID 19467154.
- ^ Bernstein H, Bernstein C, Michod RE (2012). "Chapter 1. DNA repair as the primary adaptive function of sex in bacteria and eukaryotes". In Kimura S, Shimizu S (eds.). DNA Repair: New Research. Hauppauge, N.Y.: Nova Sci. Publ. pp. 1–49. ISBN 978-1-62100-808-8.
- ^ Javaux EJ, Knoll AH, Walter MR (July 2001). "Morphological and ecological complexity in early eukaryotic ecosystems". Nature. 412 (6842): 66–69. Bibcode:2001Natur.412...66J. doi:10.1038/35083562. PMID 11452306. S2CID 205018792.
- ^ Ramesh MA, Malik SB, Logsdon JM (January 2005). "A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis". Current Biology. 15 (2): 185–191. Bibcode:2005CBio...15..185R. doi:10.1016/j.cub.2005.01.003. PMID 15668177. S2CID 17013247.
- ^ Cooper MA, Adam RD, Worobey M, Sterling CR (November 2007). "Population genetics provides evidence for recombination in Giardia". Current Biology. 17 (22): 1984–1988. Bibcode:2007CBio...17.1984C. doi:10.1016/j.cub.2007.10.020. PMID 17980591. S2CID 15991722.
- ^ Malik SB, Pightling AW, Stefaniak LM, Schurko AM, Logsdon JM (August 2007). Hahn MW (ed.). "An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis". PLOS ONE. 3 (8): e2879. Bibcode:2008PLoSO...3.2879M. doi:10.1371/journal.pone.0002879. PMC 2488364. PMID 18663385.
- ^ Dacks J, Roger AJ (June 1999). "The first sexual lineage and the relevance of facultative sex". Journal of Molecular Evolution. 48 (6): 779–783. Bibcode:1999JMolE..48..779D. doi:10.1007/PL00013156. PMID 10229582. S2CID 9441768.
- ^ Lahr DJ, Parfrey LW, Mitchell EA, Katz LA, Lara E (July 2011). "The chastity of amoebae: re-evaluating evidence for sex in amoeboid organisms". Proceedings: Biological Sciences. 278 (1715): 2081–2090. doi:10.1098/rspb.2011.0289. PMC 3107637. PMID 21429931.
- ^ Dobell, C. (1909). "Chromidia and the binuclearity hypotheses: A review and a criticism" (PDF). Quarterly Journal of Microscopical Science. 53: 279–326.
- ^ "CDC – Toxoplasmosis – Biology". 17 March 2015. Retrieved 14 June 2015.
- ^ "Cat parasite linked to mental illness, schizophrenia". CBS. 5 June 2015. Retrieved 23 September 2015.
- ^ "CDC – About Parasites". Retrieved 12 March 2013.
- ^ Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V (July 2004). "Gametocytogenesis: the puberty of Plasmodium falciparum". Malaria Journal. 3: 24. doi:10.1186/1475-2875-3-24. PMC 497046. PMID 15253774.
- ^ Tibayrenc M, et al. (June 1991). "Are eukaryotic microorganisms clonal or sexual? A population genetics vantage". Proceedings of the National Academy of Sciences of the United States of America. 88 (12): 5129–33. Bibcode:1991PNAS...88.5129T. doi:10.1073/pnas.88.12.5129. PMC 51825. PMID 1675793.
- ^ Akopyants NS, et al. (April 2009). "Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector". Science. 324 (5924): 265–268. Bibcode:2009Sci...324..265A. doi:10.1126/science.1169464. PMC 2729066. PMID 19359589.
- ^ a b c Pawlowski J, Audic S, Adl S, Bass D, Belbahri L, Berney C, Bowser SS, Cepicka I, Decelle J, Dunthorn M, Fiore-Donno AM, Gile GH, Holzmann M, Jahn R, Jirků M, Keeling PJ, Kostka M, Kudryavtsev A, Lara E, Lukeš J, Mann DG, Mitchell EAD, Nitsche F, Romeralo M, Saunders GW, Simpson AGB, Smirnov AV, Spouge JL, Stern JF, Stoeck T, Zimmermann J, Schindel D, de Vargas C (2012). "CBOL Protist Working Group: Barcoding Eukaryotic Richness beyond the Animal, Plant, and Fungal Kingdoms". PLOS Biology. 10 (11): e1001419. doi:10.1371/journal.pbio.1001419. PMC 3491025. PMID 23139639. S2CID 6330045.
- ^ Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B (2011). "How Many Species Are There on Earth and in the Ocean?". PLOS Biology. 9 (8): e1001127. doi:10.1371/journal.pbio.1001127. PMC 3160336. PMID 21886479.
- ^ Brown MW, et al. (2018), "Phylogenomics Places Orphan Protistan Lineages in a Novel Eukaryotic Super-Group", Genome Biology and Evolution, 10 (2): 427–433, doi:10.1093/gbe/evy014, PMC 5793813, PMID 29360967
- ^ a b c Eglit, Yana; Shiratori, Takashi; Jerlström-Hultqvist, Jon; Williamson, Kelsey; Roger, Andrew J.; Ishida, Ken-Ichiro; Simpson, Alastair G.B. (22 January 2024). "Meteora sporadica, a protist with incredible cell architecture, is related to Hemimastigophora". Current Biology. 34 (2): 451–459. Bibcode:2024CBio...34E.451E. doi:10.1016/j.cub.2023.12.032. PMID 38262350.
- ^ a b c d Madigan, Michael T.; Bender, Kelly S.; Buckley, Daniel H.; Sattley, W. Matthew; Stahl, David A. (2019). "Diversity of Microbial Eukarya". Brock Biology of Microorganisms (15th, Global ed.). Pearson. pp. 593–618. ISBN 9781292235103.
- ^ Simpson, Alastair G. B. (2003). "Cytoskeletal organization, phylogenetic affinities and systematics in the contentious taxon Excavata (Eukaryota)". International Journal of Systematic and Evolutionary Microbiology. 53 (6): 1759–1777. doi:10.1099/ijs.0.02578-0. PMID 14657103.
- ^ Suzuki-Tellier, Sei; Kiørboe, Thomas; Simpson, Alastair G. B. (2023). "The function of the feeding groove of 'typical excavate' flagellates". Journal of Eukaryotic Microbiology. 71 (2): e13016. doi:10.1111/jeu.13016. PMID 38108228.
- ^ Al Jewari, Caesar; Baldauf, Sandra L. (2023). "An excavate root for the eukaryote tree of life". Science Advances. 9 (17): eade4973. Bibcode:2023SciA....9E4973A. doi:10.1126/sciadv.ade4973. PMC 10146883. PMID 37115919.
- ^ a b Pánek, Tomáš; Simpson, Alastair G. B.; Brown, Matthew W.; Dyer, Betsey Dexter (2017). "Heterolobosea". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 1005–1046. doi:10.1007/978-3-319-28149-0_10. ISBN 978-3-319-28147-6.
- ^ a b c d Cavalier-Smith T (May 2022). "Ciliary transition zone evolution and the root of the eukaryote tree: implications for opisthokont origin and classification of kingdoms Protozoa, Plantae, and Fungi". Protoplasma. 259 (3): 487–593. doi:10.1007/s00709-021-01665-7. PMC 9010356. PMID 34940909.
- ^ a b Simpson, Alastair G. B. (2017). "Jakobida". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 2 (2nd ed.). Springer. pp. 973–1004. doi:10.1007/978-3-319-28149-0_6. ISBN 978-3-319-28147-6.
- ^ a b Guiry, Michael D. (2024). "How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing". Journal of Phycology. 60 (2): 214–228. Bibcode:2024JPcgy..60..214G. doi:10.1111/jpy.13431. PMID 38245909.
- ^ Kostygov, Alexei Y.; Karnkowska, Anna; Votýpka, Jan; Tashyreva, Daria; Maciszewski, Kacper; Yurchenko, Vyacheslav; Lukeš, Julius (2021). "Euglenozoa: taxonomy, diversity and ecology, symbioses and viruses". Open Biology. 11 (3): 200407. doi:10.1098/rsob.200407. PMC 8061765. PMID 33715388.
- ^ a b Adl, Sina M.; Leander, Brian S.; Simpson, Alastair G. B.; Archibald, John M.; Anderson, O. Roger; Bass, David; Bowser, Samuel S.; Brugerolle, Guy; Farmer, Mark A.; Karpov, Sergey; Kolisko, Martin; Lane, Christopher E.; Lodge, Deborah J.; Mann, David G.; Meisterfeld, Ralf; Mendoza, Leonel; Moestrup, Øjvind; Mozley-Standridge, Sharon E.; Smirnov, Alexey V.; Spiegel, Frederick (2007). "Diversity, Nomenclature, and Taxonomy of Protists". Systematic Biology. 56 (4): 684–689. doi:10.1080/10635150701494127. PMID 17661235.
- ^ Novák, Lukáš V. F.; Treitli, Sebastian C.; Pyrih, Jan; Hałakuc, Paweł; Pipaliya, Shweta V.; Vacek, Vojtěch; Brzoň, Ondřej; Soukal, Petr; Eme, Laura; Dacks, Joel B.; Karnkowska, Anna; Eliáš, Marek; Hampl, Vladimír (2023). "Genomics of Preaxostyla Flagellates Illuminates the Path Towards the Loss of Mitochondria". PLOS Genetics. 19 (12): e1011050. doi:10.1371/journal.pgen.1011050. PMC 10703272. PMID 38060519.
- ^ Eglit, Yana; Williams, Shelby K.; Roger, Andrew J.; Simpson, Alastair G.B. (2024). "Characterization of Skoliomonas gen. nov., a haloalkaliphilic anaerobe related to barthelonids (Metamonada)". Journal of Eukaryotic Microbiology: e13048. doi:10.1111/jeu.13048. PMID 39225178.
- ^ Heiss AA, Warring SD, Lukacs K, Favate J, Yang A, Gyaltshen Y, Filardi C, Simpson AGB, Kim E (December 2020). "Description of Imasa heleensis, gen. nov., sp. nov. (Imasidae, fam. nov.), a Deep-Branching Marine Malawimonad and Possible Key Taxon in Understanding Early Eukaryotic Evolution". Journal of Eukaryotic Microbiology. 68 (2): e12837. doi:10.1111/jeu.12837. PMID 33274482.
- ^ Tikhonenkov, Denis V.; Jamy, Mahwash; Borodina, Anastasia S.; Belyaev, Artem O.; Zagumyonnyi, Dmitry G.; Prokina, Kristina I.; Mylnikov, Alexander P.; Burki, Fabien; Karpov, Sergey A. (2022). "On the origin of TSAR: morphology, diversity and phylogeny of Telonemia". Open Biology. 12 (3). The Royal Society. doi:10.1098/rsob.210325. ISSN 2046-2441. PMC 8924772. PMID 35291881.
- ^ a b c d e Jirsová, Dagmar; Wideman, Jeremy G. (2024). "Integrated overview of stramenopile ecology, taxonomy, and heterotrophic origin". The ISME Journal. 18 (1): wrae150. doi:10.1093/ismejo/wrae150. PMC 11412368. PMID 39077993.
- ^ Weston EJ, Eglit Y, Simpson AG (2023). "Kaonashia insperata gen. et sp. nov., a eukaryotrophic flagellate, represents a novel major lineage of heterotrophic stramenopiles". Journal of Eukaryotic Microbiology. 71 (1): e13003. doi:10.1111/jeu.13003. PMID 37803921.
- ^ Cho, Anna; Tikhonenkov, Denis V.; Lax, Gordon; Prokina, Kristina I.; Keeling, Patrick J. (2025). "Phylogenomic position of genetically diverse phagotrophic stramenopile flagellates in the sediment-associated MAST-6 lineage and a potentially halotolerant placididean". Molecular Phylogenetics and Evolution. 190: 107964. doi:10.1016/j.ympev.2023.107964. PMID 37951557.
- ^ Mann, David G.; Crawford, Richard M.; Round, Frank E. (2017). "Bacillariophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 205–266. doi:10.1007/978-3-319-28149-0_29. ISBN 978-3-319-28147-6.
- ^ Kawai, Hiroshi; Henry, Eric C. (2017). "Phaeophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 267–304. doi:10.1007/978-3-319-28149-0_31. ISBN 978-3-319-28147-6.
- ^ Kristiansen, Jørgen; Škaloud, Pavel (2017). "Chrysophyta". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 331–366. doi:10.1007/978-3-319-28149-0_43. ISBN 978-3-319-28147-6.}
- ^ Thines M (2018). "Oomycetes". Current Biology. 28 (15): R812–R813. Bibcode:2018CBio...28.R812T. doi:10.1016/j.cub.2018.05.062. PMID 30086308.
- ^ Azuma, Tomonori; Pánek, Tomáš; Tice, Alexander K.; Kayama, Motoki; Kobayashi, Mayumi; Miyashita, Hideaki; Suzaki, Toshinobu; Yabuki, Akinori; Brown, Matthew W.; Kamikawa, Ryoma (10 April 2022). "An Enigmatic Stramenopile Sheds Light on Early Evolution in Ochrophyta Plastid Organellogenesis". Molecular Biology and Evolution. 39 (4). doi:10.1093/molbev/msac065. PMC 9004409. PMID 35348760.
- ^ Van Houten, Judith (2023). "A Review for the Special Issue on Paramecium as a Modern Model Organism". Microorganisms. 11 (4): 937. doi:10.3390/microorganisms11040937. PMC 10143506. PMID 37110360.
- ^ Lynn, Denis H. (2017). "Ciliophora". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 679–730. doi:10.1007/978-3-319-28149-0_23. ISBN 978-3-319-28147-6.
- ^ Janouškovec, Jan; Tikhonenkov, Denis V.; Mikhailov, Kirill V.; Simdyanov, Timur G.; Aleoshin, Vladimir V.; Mylnikov, Alexander P.; Keeling, Patrick J. (2013). "Colponemids Represent Multiple Ancient Alveolate Lineages". Current Biology. 23 (24): 2546–2552. Bibcode:2013CBio...23.2546J. doi:10.1016/j.cub.2013.10.062. PMID 24316202.
- ^ a b c Janouškovec, Jan; Tikhonenkov, Denis V.; Burki, Fabien; Howe, Alexis T.; Kolísko, Martin; Mylnikov, Alexander P.; Keeling, Patrick J. (2015). "Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives". PNAS. 112 (33): 10200–10207. Bibcode:2015PNAS..11210200J. doi:10.1073/pnas.1423790112. PMC 4547307. PMID 25717057.
- ^ Votýpka, Jan; Modrý, David; Oborník, Miroslav; Šlapeta, Jan; Lukeš, Julius (2017). "Apicomplexa". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 567–624. doi:10.1007/978-3-319-28149-0_20. ISBN 978-3-319-28147-6.
- ^ Itoïz, Sarah; Metz, Sebastian; Derelle, Evelyne; Reñé, Albert; Garcés, Esther; Bass, David; Soudant, Philippe; Chambouvet, Aurélie (2021). "Emerging Parasitic Protists: The Case of Perkinsea". Frontiers in Microbiology. 12: 735815. doi:10.3389/FMICB.2021.735815. PMC 8792838. PMID 35095782.
- ^ a b Saldarriaga, Juan F.; Taylor, F. J. R. 'Max' (2017). "Dinoflagellata". In Archibald, John M.; Simpson, Alastair G.B.; Slamovits, Claudio H. (eds.). Handbook of the Protists. Vol. 1 (2nd ed.). Springer. pp. 625–678. doi:10.1007/978-3-319-28149-0_22. ISBN 978-3-319-28147-6.
- ^ Waller, Ross F.; Kořený, Ludĕk (2017). "Plastid Complexity in Dinoflagellates: A Picture of Gains, Losses, Replacements and Revisions". Advances in Botanical Research. 84: 105–143. doi:10.1016/bs.abr.2017.06.004. ISBN 978-0-12-802651-9.
- ^ Cavalier-Smith; Chao; Lewis (2015), "Multiple origins of Heliozoa from flagellate ancestors: New cryptist subphylum Corbihelia, superclass Corbistoma, and monophyly of Haptista, Cryptista, Hacrobia and Chromista", Molecular Phylogenetics and Evolution, 93: 331–362, Bibcode:2015MolPE..93..331C, doi:10.1016/j.ympev.2015.07.004, PMID 26234272
- ^ Yazaki, Euki; Yabuki, Akinori; Imaizumi, Ayaka; Kume, Keitaro; Hashimoto, Tetsuo; Inagaki, Yuji (2022). "The closest lineage of Archaeplastida is revealed by phylogenomics analyses that include Microheliella maris". Open Biol. 12 (4): 210376. doi:10.1098/rsob.210376. PMC 9006020. PMID 35414259.
- ^ a b Olivier De Clerck; Kenny A. Bogaert; Frederik Leliaert (2012). "Chapter Two – Diversity and Evolution of Algae: Primary Endosymbiosis". In Gwenaël Piganeau (ed.). Advances in Botanical Research. Vol. 64. Academic Press. pp. 55–86. doi:10.1016/B978-0-12-391499-6.00002-5. ISBN 9780123914996. ISSN 0065-2296.
- ^ a b Gawryluk, Ryan M. R.; Tikhonenkov, Denis V.; Hehenberger, Elisabeth; Husnik, Filip; Mylnikov, Alexander P.; Keeling, Patrick J. (August 2019). "Non-photosynthetic predators are sister to red algae". Nature. 572 (7768): 240–243. doi:10.1038/s41586-019-1398-6. ISSN 1476-4687. PMID 31316212. S2CID 197542583.
- ^ Tedersoo, Leho; Sánchez-Ramírez, Santiago; Kõljalg, Urmas; Bahram, Mohammad; Döring, Markus; Schigel, Dmitry; May, Tom; Ryberg, Martin; Abarenkov, Kessy (2018), "High-level classification of the Fungi and a tool for evolutionary ecological analyses", Fungal Diversity, 90: 135–159, doi:10.1007/s13225-018-0401-0, hdl:10138/238983, S2CID 21714270
- ^ Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; et al. (2022). "Outline of Fungi and fungus-like taxa – 2021". Mycosphere. 13 (1): 53–453. doi:10.5943/mycosphere/13/1/2. hdl:10481/76378. S2CID 249054641.
- ^ Tikhonenkov DV, Mikhailov KV, Hehenberger E, Mylnikov AP, Aleoshin VV, Keeling PJ, et al. (2020). "New Lineage of Microbial Predators Adds Complexity to Reconstructing the Evolutionary Origin of Animals". Current Biology. 30 (22): 4500–4509. Bibcode:2020CBio...30E4500T. doi:10.1016/j.cub.2020.08.061. PMID 32976804.
- ^ Torruella G, Galindo LJ, Moreira D, Ciobanu M, Heiss AA, Yubuki N, et al. (2022). "Expanding the molecular and morphological diversity of Apusomonadida, a deep-branching group of gliding bacterivorous protists". Journal of Eukaryotic Microbiology. 70 (2): e12956. doi:10.1111/jeu.12956. hdl:2117/404026. PMID 36453005. S2CID 253460648.
- ^ Lax, Gordon; Eglit, Yana; Eme, Laura; Bertrand, Erin M.; Roger, Andrew J.; Simpson, Alastair G. B. (14 November 2018). "Hemimastigophora is a novel supra-kingdom-level lineage of eukaryotes". Nature. 564 (7736): 410–414. Bibcode:2018Natur.564..410L. doi:10.1038/s41586-018-0708-8. ISSN 0028-0836. PMID 30429611. S2CID 205570993.
- ^ Bar-On, Yinon M.; Phillips, Rob; Milo, Ron (17 May 2018). "The biomass distribution on Earth". Proceedings of the National Academy of Sciences. 115 (25): 6506–6511. Bibcode:2018PNAS..115.6506B. doi:10.1073/pnas.1711842115. ISSN 0027-8424. PMC 6016768. PMID 29784790.
- ^ a b c d e f g Singer, David; Seppey, Christophe V.W.; Lentendu, Guillaume; Dunthorn, Micah; Bass, David; Belbahri, Lassâad; Blandenier, Quentin; Debroas, Didier; de Groot, G. Arjen; de Vargas, Colomban; Domaizon, Isabelle; Duckert, Clément; Izaguirre, Irina; Koenig, Isabelle; Mataloni, Gabriela; Schiaffino, M. Romina; Mitchell, Edward A.D.; Geisen, Stefan; Lara, Enrique (January 2021). "Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems". Environment International. 146 (106262): 106262. Bibcode:2021EnInt.14606262S. doi:10.1016/j.envint.2020.106262. hdl:10261/265020. PMID 33221595.
- ^ Keeling PJ, Campo JD (June 2017). "Marine Protists Are Not Just Big Bacteria". Current Biology. 27 (11): R541–R549. Bibcode:2017CBio...27.R541K. doi:10.1016/j.cub.2017.03.075. PMID 28586691.
- ^ a b c d Faure, Emile; Not, Fabrice; Benoiston, Anne-Sophie; Labadie, Karine; Bittner, Lucie; Ayata, Sakina-Dorothée (April 2019). "Mixotrophic protists display contrasted biogeographies in the global ocean". The ISME Journal. 13 (4): 1072–1083. Bibcode:2019ISMEJ..13.1072F. doi:10.1038/s41396-018-0340-5. PMC 6461780. PMID 30643201.
- ^ Epstein, Slava; López-García, Purificación (2007). ""Missing" protists: a molecular perspective". Biodiversity and Conservation. 17 (2): 261–276. doi:10.1007/s10531-007-9250-y. S2CID 3960288.
- ^ Leles SG, Mitra A, Flynn KJ, Stoecker DK, Hansen PJ, Calbet A, McManus GB, Sanders RW, Caron DA, Not F, Hallegraeff GM, Pitta P, Raven JA, Johnson MD, Glibert PM, Våge S (August 2017). "Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance". Proceedings of the Royal Society B: Biological Sciences. 284 (1860): 20170664. doi:10.1098/rspb.2017.0664. PMC 5563798. PMID 28768886.
- ^ a b Mitra, Aditee; Flynn, Kevin J.; Tillmann, Urban; Raven, John A.; Caron, David; Stoecker, Diane K.; Not, Fabrice; Hansen, Per J.; Hallegraeff, Gustaaf; Sanders, Robert; Wilken, Susanne; McManus, George; Johnson, Mathew; Pitta, Paraskevi; Våge, Selina; Berge, Terje; Calbet, Albert; Thingstad, Frede; Jeong, Hae Jin; Burkholder, JoAnn; Glibert, Patricia M.; Granéli, Edna; Lundgren, Veronica (2016). "Defining Planktonic Protist Functional Groups on Mechanisms for Energy and Nutrient Acquisition: Incorporation of Diverse Mixotrophic Strategies". Protist. 167 (2): 106–120. doi:10.1016/j.protis.2016.01.003. hdl:10261/131722. PMID 26927496.
- ^ Metz S, Huber P, Accattatis V, Lopes dos Santos A, Bigeard E, Unrein F, Chambouvet A, Not F, Lara E, Devercelli M (2022). "Freshwater protists: unveiling the unexplored in a large floodplain system". Environmental Microbiology. 24 (4): 1731–1745. Bibcode:2022EnvMi..24.1731M. doi:10.1111/1462-2920.15838. PMID 34783136. S2CID 244133100.
- ^ a b c d e f Geisen, Stefan; Mitchell, Edward A. D.; Adl, Sina; Bonkowski, Michael; Dunthorn, Micah; Ekelund, Flemming; Fernández, Leonardo D.; Jousset, Alexandre; Krashevska, Valentyna; Singer, David; Spiegel, Frederick W.; Walochnik, Julia; Lara, Enrique (May 2018). "Soil protists: a fertile frontier in soil biology research". FEMS Microbiology Reviews. 42 (3): 293–323. doi:10.1093/femsre/fuy006. PMID 29447350.
- ^ Harder C, Rønn R, Brejnrod A, et al. (8 March 2016). "Local diversity of heathland Cercozoa explored by in-depth sequencing". The ISME Journal. 10 (10): 2488–2497. Bibcode:2016ISMEJ..10.2488H. doi:10.1038/ismej.2016.31. PMC 5030685. PMID 26953604.
- ^ Geisen, Stefan; Koller, Robert; Hünninghaus, Maike; Dumack, Kenneth; Urich, Tim; Bonkowski, Michael (2016). "The soil food web revisited: Diverse and widespread mycophagous soil protists". Soil Biology and Biochemistry. 94: 10–18. Bibcode:2016SBiBi..94...10G. doi:10.1016/j.soilbio.2015.11.010.
- ^ Schwelm A, Badstöber J, Bulman S, Desoignies N, Etemadi M, Falloon RE, Gachon CM, Legreve A, Lukeš J, Merz U, Nenarokova A, Strittmatter M, Sullivan BK, Neuhauser S (April 2018). "Not in your usual Top 10: protists that infect plants and algae". Molecular Plant Pathology. 19 (4): 1029–1044. doi:10.1111/mpp.12580. PMC 5772912. PMID 29024322.
- ^ Kamoun S, Furzer O, Jones JD, Judelson HS, Ali GS, Dalio RJ, Roy SG, Schena L, Zambounis A, Panabières F, Cahill D, Ruocco M, Figueiredo A, Chen XR, Hulvey J, Stam R, Lamour K, Gijzen M, Tyler BM, Grünwald NJ, Mukhtar MS, Tomé DF, Tör M, Van Den Ackerveken G, McDowell J, Daayf F, Fry WE, Lindqvist-Kreuze H, Meijer HJ, Petre B, Ristaino J, Yoshida K, Birch PR, Govers F (May 2015). "The Top 10 oomycete pathogens in molecular plant pathology". Molecular Plant Pathology. 16 (4): 413–34. doi:10.1111/mpp.12190. PMC 6638381. PMID 25178392.
- ^ Campbell, N. and Reese, J. (2008) Biology. Pearson Benjamin Cummings; 8 ed. ISBN 0805368442. pp. 583, 588
- ^ Lauckner, G. (1980). "Diseases of protozoa". In: Diseases of Marine Animals. Kinne, O. (ed.). Vol. 1, p. 84, John Wiley & Sons, Chichester, UK.
- ^ Cox, F.E.G. (1991). "Systematics of parasitic protozoa". In: Kreier, J.P. & J. R. Baker (ed.). Parasitic Protozoa, 2nd ed., vol. 1. San Diego: Academic Press.
- ^ Keen EC (July 2013). "Beyond phage therapy: virotherapy of protozoal diseases". Future Microbiology. 8 (7): 821–3. doi:10.2217/FMB.13.48. PMID 23841627.
- ^ Hyman P, Atterbury R, Barrow P (May 2013). "Fleas and smaller fleas: virotherapy for parasite infections". Trends in Microbiology. 21 (5): 215–220. doi:10.1016/j.tim.2013.02.006. PMID 23540830.
- ^ Liu YJ, Hodson MC, Hall BD (September 2006). "Loss of the flagellum happened only once in the fungal lineage: phylogenetic structure of kingdom Fungi inferred from RNA polymerase II subunit genes". BMC Evolutionary Biology. 6: 74. doi:10.1186/1471-2148-6-74. PMC 1599754. PMID 17010206.
- ^ "ARS Parasite Collections Assist Research and Diagnoses". USDA Agricultural Research Service. January 28, 2010.
- ^ Durham, Sharon (January 28, 2010) ARS Parasite Collections Assist Research and Diagnoses. Ars.usda.gov. Retrieved 2014-03-20.
- ^ a b Barry S. C. Leadbeater; Sharon M. M. McReady (2000). "Chapter 1. The flagellates: historical perspectives". In Barry S. C. Leadbeater; J. C. Green (eds.). The Flagellates. Unity, diversity and evolution. London: Taylor & Francis. pp. 1–26. doi:10.1201/9781482268225. ISBN 9780429182136.
- ^ Marc J. Ratcliff (2009). "The Emergence of the Systematics of Infusoria". The Quest for the Invisible: Microscopy in the Enlightenment. Ashgate. pp. 177–216. ISBN 9781409480266.
- ^ Goldfuß (1818). "Ueber die Classification der Zoophyten" [On the classification of zoophytes]. Isis, Oder, Encyclopädische Zeitung von Oken (in German). 2 (6): 1008–1019. From p. 1008: "Erste Klasse. Urthiere. Protozoa." (First class. Primordial animals. Protozoa.) [Note: each column of each page of this journal is numbered; there are two columns per page.]
- ^ Carl Theodor Ernst von Siebold; Hermann Stannius (1846–1848). Lehrbuch der vergleichenden Anatomie Vol. 1: Wirbellose Thiere [Textbook of Comparative Anatomy Vol. 1: Invertebrate Animals] (in German). Vol. 1. Berlin, Germany: Veit. p. 3. p. 3:
Erste Hauptgruppe. Protozoa. Thiere, in welchen die verschiedenen Systeme der Organe nicht scharf ausgeschieden sind, und deren unregelmässige Form und einfache Organisation sich auf eine Zelle reduziren lassen.
[First principal group. Protozoa. Animals, in which the different systems of organs are not sharply separated, and whose irregular form and simple organization can be reduced to one cell.] - ^ John Hogg (1860). "On the distinctions of a Plant and an Animal, and on a Fourth Kingdom of Nature". Edinburgh New Philosophical Journal. 2nd series. 12: 216–225. p. 223:
... I here suggest a fourth or an additional kingdom, under the title of the Primigenal kingdom, ... This Primigenal kingdom would comprise all the lower creatures, or the primary organic beings, – 'Protoctista,' – from πρώτος, first, and χτιστά, created beings; ...
- ^ Haeckel, Ernst (1878). Das protistenreich. Eine populäre uebersicht über das formengebiet der niedersten lebewesen [The protistan kingdom. A popular survey of the forms of the lowest living beings] (in German). Leipzig: E. Günther. doi:10.5962/bhl.title.58542.
- ^ Taylor, F. J. R. 'Max' (11 January 2003). "The collapse of the two-kingdom system, the rise of protistology and the founding of the International Society for Evolutionary Protistology (ISEP)". International Journal of Systematic and Evolutionary Microbiology. 53 (6): 1707–1714. doi:10.1099/ijs.0.02587-0. PMID 14657097.
- ^ Haeckel, Ernst (1866). Generelle Morphologie der Organismen [The General Morphology of Organisms] (in German). Vol. 1. Berlin, (Germany): G. Reimer. pp. 215ff. From p. 215: "VII. Character des Protistenreiches." (VII. Character of the kingdom of Protists.)
- ^ Rothschild, Lynn J. (1989). "Protozoa, Protista, Protoctista: what's in a name?". Journal of the History of Biology. 22 (2): 277–305. doi:10.1007/BF00139515. PMID 11542176. S2CID 32462158.
- ^ Copeland HF (1938). "The Kingdoms of Organisms". Quarterly Review of Biology. 13 (4): 383–420. doi:10.1086/394568. JSTOR 2808554. S2CID 84634277.
- ^ a b Whittaker RH (1959). "On the Broad Classification of Organisms". Quarterly Review of Biology. 34 (3): 210–226. doi:10.1086/402733. JSTOR 2816520. PMID 13844483. S2CID 28836075.
- ^ Whittaker RH (January 1969). "New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms". Science. 163 (3863): 150–160. Bibcode:1969Sci...163..150W. CiteSeerX 10.1.1.403.5430. doi:10.1126/science.163.3863.150. PMID 5762760.
- ^ Hagen JB (2012). "depiction of Whittaker's early four-kingdom system, based on three modes of nutrition and the distinction between unicellular and multicellular body plans". BioScience. 62: 67–74. doi:10.1525/bio.2012.62.1.11.
- ^ Margulis L, Chapman MJ (2009-03-19). Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Academic Press. ISBN 9780080920146.
- ^ Archibald, John M.; Simpson, Alastair G. B.; Slamovits, Claudio H., eds. (2017). Handbook of the Protists (2 ed.). Springer International Publishing. pp. ix. ISBN 978-3-319-28147-6.
- ^ Stechmann A, Cavalier-Smith T (September 2003). "The root of the eukaryote tree pinpointed" (PDF). Current Biology. 13 (17): R665–667. Bibcode:2003CBio...13.R665S. doi:10.1016/S0960-9822(03)00602-X. PMID 12956967. S2CID 6702524.
- ^ Schlegel, M.; Hulsmann, N. (2007). "Protists – A textbook example for a paraphyletic taxon☆". Organisms Diversity & Evolution. 7 (2): 166–172. Bibcode:2007ODivE...7..166S. doi:10.1016/j.ode.2006.11.001.
- ^ "Protista". microbeworld.org. Archived from the original on 13 June 2016. Retrieved 11 June 2016.
- ^ Štolc A (1899). "Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies". Bull. Int. l'Acad. Sci. Bohème. 12: 1–12.
- ^ Adl SM, Simpson AG, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF (2005). "The new higher level classification of eukaryotes with emphasis on the taxonomy of protists". The Journal of Eukaryotic Microbiology. 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873. S2CID 8060916.
- ^ O'Malley, Maureen A. (2022). "Getting at the Basics of Multicellularity". In Herron, Matthew D.; Conlin, Peter L.; Ratcliff, William C. (eds.). The Evolution of Multicellularity. Evolutionary Cell Biology (1st ed.). CRC Press. pp. 9–24. doi:10.1201/9780429351907. ISBN 9780429351907. S2CID 248578172.
- ^ a b Ruggiero, Michael A.; Gordon, Dennis P.; Orrell, Thomas M.; Bailly, Nicolas; Bourgoin, Thierry; Brusca, Richard C.; Cavalier-Smith, Thomas; Guiry, Michael D.; Kirk, Paul M.; Thuesen, Erik V. (2015). "A higher level classification of all living organisms". PLOS ONE. 10 (4): e0119248. Bibcode:2015PLoSO..1019248R. doi:10.1371/journal.pone.0119248. PMC 4418965. PMID 25923521.
- ^ a b c d e f Brocks, Jochen J.; Nettersheim, Benjamin J.; Adam, Pierre; Schaeffer, Philippe; Jarrett, Amber J. M.; Güneli, Nur; Liyanage, Tharika; van Maldegem, Lennart M.; Hallmann, Christian; Hope, Janet M. (2023). "Lost world of complex life and the late rise of the eukaryotic crown" (PDF). Nature. 618 (7966): 767–773. Bibcode:2023Natur.618..767B. doi:10.1038/s41586-023-06170-w. PMID 37286610. S2CID 259111647.
- ^ Porter SM, Meisterfeld R, Knoll AH (May 2003). "Vase-shaped microfossils from the Neoproterozoic Chuar Group, Grand Canyon: a classification guided by modern testate amoebae" (PDF). Journal of Paleontology. 77 (3): 409–429. Bibcode:2003JPal...77..409P. doi:10.1666/0022-3360(2003)077<0409:VMFTNC>2.0.CO;2.
- ^ Parfrey LW, Lahr DJ, Knoll AH, Katz LA (August 2011). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks". Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13624–9. Bibcode:2011PNAS..10813624P. doi:10.1073/pnas.1110633108. PMC 3158185. PMID 21810989.
- ^ Chang, Shan; Feng, Qinglai; Zhang, Lei (14 August 2018). "New Siliceous Microfossils from the Terreneuvian Yanjiahe Formation, South China: The Possible Earliest Radiolarian Fossil Record". Journal of Earth Science. 29 (4): 912–919. Bibcode:2018JEaSc..29..912C. doi:10.1007/s12583-017-0960-0. S2CID 134890245.
- ^ Zhang, Ke; Feng, Qing-Lai (September 2019). "Early Cambrian radiolarians and sponge spicules from the Niujiaohe Formation in South China". Palaeoworld. 28 (3): 234–242. doi:10.1016/j.palwor.2019.04.001. S2CID 146452469.
- ^ Maletz, Jörg (June 2017). "The identification of putative Lower Cambrian Radiolaria". Revue de Micropaléontologie. 60 (2): 233–240. Bibcode:2017RvMic..60..233M. doi:10.1016/j.revmic.2017.04.001.
Bibliography
[edit]General
[edit]- Hausmann, K., N. Hulsmann, R. Radek. Protistology. Schweizerbart'sche Verlagsbuchshandlung, Stuttgart, 2003.
- Margulis, L., J.O. Corliss, M. Melkonian, D.J. Chapman. Handbook of Protoctista. Jones and Bartlett Publishers, Boston, 1990.
- Margulis, L., K.V. Schwartz. Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth, 3rd ed. New York: W.H. Freeman, 1998.
- Margulis, L., L. Olendzenski, H.I. McKhann. Illustrated Glossary of the Protoctista, 1993.
- Margulis, L., M.J. Chapman. Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Amsterdam: Academic Press/Elsevier, 2009.
- Schaechter, M. Eukaryotic microbes. Amsterdam, Academic Press, 2012.
Physiology, ecology and paleontology
[edit]- Fontaneto, D. Biogeography of Microscopic Organisms. Is Everything Small Everywhere? Cambridge University Press, Cambridge, 2011.
- Moore, R. C., and other editors. Treatise on Invertebrate Paleontology. Protista, part B (vol. 1[permanent dead link], Charophyta, vol. 2, Chrysomonadida, Coccolithophorida, Charophyta, Diatomacea & Pyrrhophyta), part C (Sarcodina, Chiefly "Thecamoebians" and Foraminiferida) and part D[permanent dead link] (Chiefly Radiolaria and Tintinnina). Boulder, Colorado: Geological Society of America; & Lawrence, Kansas: University of Kansas Press.
External links
[edit]- UniEuk Taxonomy App
- Tree of Life: Eukaryotes
- Tsukii, Y. (1996). Protist Information Server (database of protist images). Laboratory of Biology, Hosei University. Protist Information Server. Updated: March 22, 2016.















